10 U.S. policy ideas for 10 lifesaving technologies

Tom Kalil responded to my first blog post on 10 technologies that won’t exist in 5 years, asking: OK, how do we make them happen? Ashish Jha responded saying we as a society can decide to make new lifesaving technologies, and it’s a policy choice:
True
— Ashish K. Jha, MD, MPH (@ashishkjha) February 5, 2024
We CAN save millions of lives through technologies, vaccines and treatments to things that kill lots of people
But we're not organized for it
We could be. Operation Warp Speed was one example
But we see OWS as a one-off, not as model for solving big scientific problems https://t.co/kafTykCGEl
Between the two of them, Kalil and Jha were science advisors in three different White Houses, so I wanted to take some time to reflect on their prompts, then respond. I agree with Jha, and answering Kalil would take more than a blog post. So I'm starting with a smaller question: what can the U.S. government do to help these 10 technologies exist?
This first post contains the first 5 policy ideas. I have two filters for ideas, which both must be met:
- Would the policy change concretely help for developing any of the 10 technologies listed, in the near term? (Straightaway/next few years)
- Is the idea a step towards a world that makes more sense, where people affected by any health problem have more options and autonomy? (Next few decades)
My day job is to fund scientists, and think about science. I have never worked in the White House, the NIH, the FDA, BARDA, nor the CDC, nor on a Congressional appropriations committee. I’ve never lived in D.C., though I’ve visited the Lincoln Memorial. Probably several of the ideas below are bad for reasons I don’t understand. If you’re reading this at one of the agencies and wondering “who the heck is he?”, the answer is: one citizen with 10 ideas. Thank you for your service!
[This post is written in my personal capacity, not as part of my job, does not represent the views of my employer, etc.]
Congress
1. [$billions needed for this one] Renew Gavi and the Global Fund at a high level in the upcoming replenishments. “Fund this” is a boring way to start a list, but this one is probably the most important, so I’m putting it up top. Gavi provides clear demand in public markets for vaccines to benefit people in lower- and middle-income countries; Gavi would be the main payer in a lot of countries for #1, #2, and #10 on the list, were each to be invented (TB vaccine for adults, strep A vaccine, syphilis vaccine). The Global Fund serves a similar function for drugs and diagnostics and other malaria/TB/HIV products; they’re a possible payer for #3, #4, and #8 (malaria monoclonal antibody, bugs to stop malaria, a single test to distinguish causes of fever). This pooled demand means groups making new health products know they’ll be able to sell the products if they succeed, in countries where the need is high but ability to pay is low.
There is a relatively clear case that our contribution to Gavi should increase this cycle because of new vaccines that just got approved or are likely to be approved in the next 5 year window that will otherwise go unused – the finally-we-have them malaria vaccines, Shigella, dengue, and more. These first generation malaria vaccines won’t get rid of the disease on their own, but I’m quite nervous that ambition is not set high enough on hurrying up and getting them to more kids. A careful comment from Gavi two days ago made me shudder at the track we're on: “It is accurate to say that we are moving from a [vaccine] supply constrained environment to a resource constrained one.” Are we, as a country, as a world, really going to let money be the blocker to kids getting a malaria vaccine?
Decades hence, the U.S. will not need to contribute much to Gavi, as lower income country economies grow. (That growth assumes productive U.S. trade and development policies beyond this post.) Domestic governments contribute at least 20 cents to every vaccine dose today, and once they’re rich enough they will take over the full cost; obsolescence is built into the system. Today, let's get there on the basics.
2. [No new appropriations (or negligible) needed for this change.] Create an application track for Neglected Tropical Disease drugs that ends with the FDA proactively sharing its assessments unredacted, for faster drug approval decisions in lower- and middle-income countries.
Currently the FDA is in an awkward middle ground. Neglected Tropical Disease (NTD) drugs often get a sped up review process (great). And there’s a voucher system that provides some economic incentive to develop neglected drugs that no other country has, including the European Union (also great). Once you get approved in the U.S., and win a voucher, you still have to get your drug approved in the countries where e.g. malaria is actually prevalent too – just like the FDA has to approve all drugs used here (makes sense, but perhaps you can see trouble brewing).
The Europeans don't have a voucher system (bad) – but they have an approval track for products that are mostly going to be used elsewhere. If you apply using that track, they loop in regulators from those countries too. They share the documents assessing your clinical data and inspecting your manufacturing site with the WHO prequalification (PQ) team – the team whose stamp of approval speeds things up for many countries with less experienced national regulators. Gavi and the Global Fund need a product to be prequalified in order to buy it through the UN procurement agencies (e.g. UNICEF, for children’s vaccines). They always need the product to be approved in the country it’s going to be used, even in the rare cases they pre-empt or skirt the UN.
The FDA has no such track. We don't loop others in along the way by default, or share our assessments of why we've made an approval decision until those assessments go online. Once they're online, they look like this:

"(b)(4)" refers to an exemption under the Freedom of Information Act, used to redact confidential information, often private business information or trade secrets. The social merits of that part of the law are debatable in general, but for tropical diseases no one wants this to happen. The companies must be annoyed by it too since it means it takes longer to get their products approved in the places they’re mostly going to be sold.
That screenshot is from one of the assessment documents for fexinidazole, a drug for treating African trypanosomiasis, otherwise known as sleeping sickness. You get sleeping sickness from tsetse fly bites. We don't have tsetse flies here – well, not since the Paleogene. Over half of reported sleeping sickness cases are in the Democratic Republic of Congo. How helpful do you think the DRC health regulator finds that report?
You may be wary I'm cherry-picking a paragraph that looks bad from somewhere in the middle of the report. It's true, that screenshot was from page 15. Here's an excerpt from pages 3 and 4:

Our current approval system is basically set up to communicate: we have the expertise to assess these NTD drugs, and we're going to assess them quickly (Priority Review) – but once we approve them we're not telling anyone why we did it, anyone who needs the details to act on it.
So: let's create a track for products on the NTD list, sharing assessments with few or no redactions with the WHO PQ system, and allow PQ to share those documents further with regulators in partner countries. The NTD list already exists, precisely for diseases that aren't common in the U.S. As a bonus, this change would help address concerns that priority review vouchers for NTDs don't lead to drugs being accessible quickly enough, even if they lead to more approvals. Let’s make the system smoother, avoid duplication, and speed up availability of new products where they are most needed – sometimes by several years.
(FDA might be able to set up a path like this on its own, without Congress. I'm not a lawyer. Since the path would also interact with the voucher program, and with user fees, I figured Congress may be helpful for clarity and confidence either way.)
((Why only NTD products? There should be more sharing of information on other drug reviews too, when another regulator asks, even if the information is not all public. More in a future post.))
3. [No new appropriations (or negligible) needed for this change.] Keep PEPFAR on 5 year budget cycles not 1 year renewals. PEPFAR is a U.S. government success story, and needs operational flexibility to plan ahead.
HIV/AIDS still kills 600,000 people, but this number is being driven down largely thanks to PEPFAR:
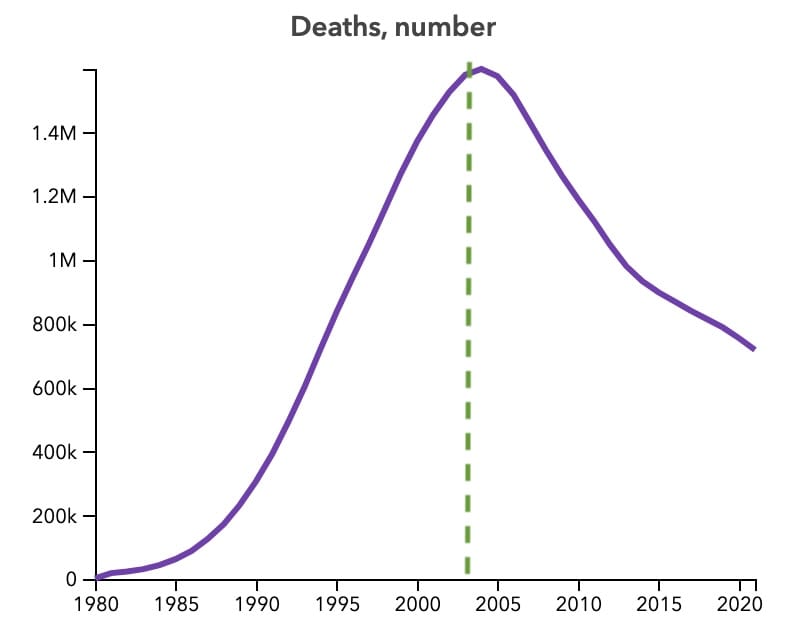
I’ve worked at a 4-person organisation, and we started having serious meetings when we had only one year of visibility in funding. Imagine there are thousands of you in tens of countries managing billions of dollars in programs. And your job is to help provide reliable health infrastructure to vulnerable people. A funder letting you know what’s available in one year cycles is irresponsible.
The benefits of PEPFAR programs mostly reach HIV/AIDS patients, but the reason I’ve included this policy change as helpful for the 10 unmade technologies too is that many AIDS deaths in Southern Africa are caused by TB infections, and syphilis is also a coinfection that can make HIV infections more dangerous (and vice versa). Having a vaccine for either would help. Many of the sites that are able to run clinical trials for TB vaccines have PEPFAR to thank for their strength and experience. Those sites have tested drugs that have gone on to benefit people living with HIV in the U.S. too; next, maybe they can help get the U.S. outbreaks of syphilis under control.
4. [No new appropriations (or negligible) needed for this change.] Consider legislation to introduce Transferable Exclusivity Vouchers for Neglected Tropical Disease drugs. Priority Review Vouchers are already transferable in that you can sell them to another company if you win one. PRVs are all well and good, but R&D is expensive enough that $100M isn’t a sufficiently large carrot to promote research on some problems – you could get a $100M PRV for an approved TB vaccine, but the pre-approval phase 3 trial may cost you $550M.
Transferable Exclusivity Vouchers could provide a 5 to 10 times more powerful incentive than Priority Review Vouchers, depending how they are designed. That’s because they (a) could be sold to companies with already approved patented drugs (rather than drugs going up for review with uncertainty on the outcome and eventual sales), and (b) you can only realistically shave 4 months off a 10 month review process whereas you could can extend exclusivity by 1 month, 6 months, 12 months – whatever Congress decides is the right balance.
The devil is in the details on how these voucher systems get designed, though – in particular, when changing timelines on exclusivity you have to be careful that while helping people avoid TB you don’t hurt uninsured people who need patented diabetes medicines and have to pay out of pocket for longer. At the end of the day, exclusivity never feels good, even if it’s what induces much of the innovation in our current system. Vouchers are a useful tool if you want to change the incentives of what gets invented to help more patients, while leaving the inventing to companies with shareholders. That’s not nothing.
5. [$100s of millions needed for this one.] Increase funding for the NIH’s Fogarty International Center, as recommended by the Global Health Technologies Coalition. If you want discoveries, you need discoverers; inventions, inventors. Fogarty funds the strengthening of a scientific workforce in lower- and middle-income countries. Really, the spread of scientific culture, the creation of scientists. That's through e.g. awarding postdocs for African students to spend 2 years at the NIH then 2 years in their home institutions, and offering research training on infectious diseases where you can get your first experience helping run a clinical trial.
In Brazil, scientists discovered the link between Zika and brain damage. In West Africa, scientists helped contain the Ebola outbreak to Guinea, Liberia, and Sierra Leone. In Southern Africa, scientists led the trials that determined getting on HIV treatment reduces transmission, bringing peace to millions of HIV positive people and their uninfected partners – including in the U.S. Those scientists were working at universities and clinical research companies and non-profits and public health institutions when they made their discoveries. You don't wake up one day able to do that job; it takes years of training. Without Fogarty, we'd all be more at risk.
Fogarty's budget is ~$100M per year right now, i.e. <1% of the NIH’s total budget, with outsized returns. Expansion would, probably, help development of all 10 of the technologies – here and there, one young scientist at a time.
Your post has been split into two parts to ease the download. Stay tuned for part 2, on the FDA, CDC, ARPA-H…
Thanks to Tom Kalil and Javier Guzman for comments, and to various science & health policy experts who have educated me on these topics over the years